www.medical-devices.tech
12
'25
Written on Modified on
Smith+Nephew to highlight breakthrough Sports Medicine technologies for joint repair at AAOS 2025
Smith+Nephew will showcase its latest Sports Medicine advancements for joint repair at the AAOS Annual Meeting in San Diego.
www.smith-nephew.com
Smith+Nephew, the global medical technology company, today announces it will showcase the latest advancements in Sports Medicine for joint repair at the American Academy of Orthopaedic Surgeons Annual Meeting in San Diego this week. Some of the highlighted technologies will include:
Spatial Surgery
Smith+Nephew continues to pioneer in Sports Medicine and is excited to introduce a new category called Spatial Surgery - a revolutionary new frontier in arthroscopic surgical innovation. This 510(k)-pending technology called the TESSA Spatial Surgery System (Tracking Enabled Spatial Surgery Assistant) plans to combine personalized operative planning with a real-time, tracking enabled device using advanced imaging and augmented reality guidance to assist a surgeon in decision making.
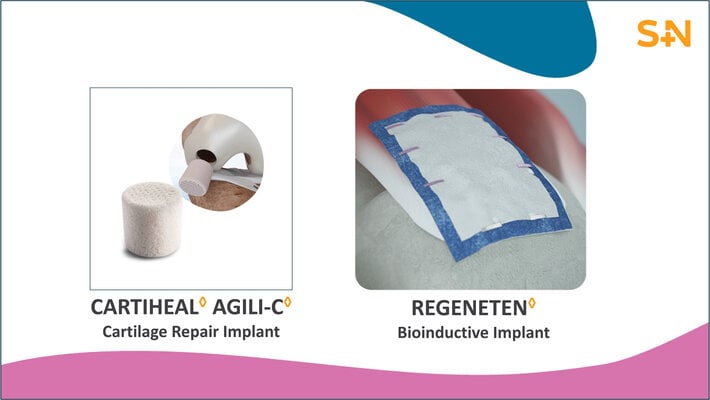
CARTIHEAL AGILI-C Cartilage Repair Implant
Smith+Nephew’s CARTIHEAL AGILI-C Cartilage Repair Implant is an FDA approved device that was previously granted breakthrough designation and is now evolving the cartilage repair landscape. The unique properties of the implant enable physicians to surgically treat patients that previously had no access to cartilage repair procedures. In a large randomized controlled trial, when compared to the surgical standard of care, the CARTIHEAL AGILI-C implant demonstrated:
- Proven Clinical Superiority: Patients treated with the CARTIHEAL AGILI-C Implant reported significantly better knee function, pain relief, and mobility improvements over a 4-year period.
- Significantly lower risk of TKA or osteotomy: Patients’ risk of additional knee reconstruction/realignment surgery at 4 years.
- Different Patient Profiles – Same Great Results: The scaffold treats a broad group of patients across age, lesion size, and presence of osteoarthritis while delivering clinically meaningful results.
REGENETEN Bioinductive Implant
The REGENETEN Bioinductive Implant is a scaffold made from highly purified type I collagen fibers and has changed the way surgeons treat tendon injuries for more than ten years. Clinical studies in rotator cuff repair have demonstrated results that surpass the current standard of care:
The REGENETEN Bioinductive Implant is a scaffold made from highly purified type I collagen fibers and has changed the way surgeons treat tendon injuries for more than ten years. Clinical studies in rotator cuff repair have demonstrated results that surpass the current standard of care:
- 3x reduction in the risk of re-tear in a randomized controlled trial versus standard repair alone augmenting repair of medium-to-large full-thickness tears (at 1-year).
- Accelerated recovery and return to activity when used as an isolated treatment (compared with takedown and suture anchor repair), while leading to consistent tendon healing for partial-thickness tears.
- Well established technique used in >150,000 patients, with a proprietary delivery and fixation system resulting in a 15-minute procedure.
In addition to continued value in rotator cuff repair, usage of the REGENETEN Implant continues to increase in tendons around the body, including those in the hip, knee and foot & ankle, where surgeons recognize its potential to improve tendon healing.
www.smith-nephew.com
www.smith-nephew.com


